This post is also available in: 简体中文 (Chinese (Simplified))
Hydrogen-based Fine-Ore Reduction (or HYFOR for short) is the world’s first direct-reduction process for iron-ore concentrates from ore beneficiation that does not require any preprocessing of the material like sintering or pelletizing. Building on comprehensive experience from Primetals Technologies’ Finmet and Finex processes, the new technology can be applied to all types of beneficiated ore. It works with particle sizes of less than 0.15 mm for 100 percent of the feedstock, while allowing a maximum grain size of 0.5 mm. Thanks to the large particle surface, the process achieves high reduction rates at low temperatures and pressures.
This article was originally published in Metals Magazine #09 (Jan 2020) and updated in September 2021 to reflect new developments.
- H2 as a reducing agent
- Direct use of iron-ore concentrate fines
- No agglomeration
- Modular plant design
Hydrogen or hydrogen-rich gases
As a primary reduction agent, the new process uses H2 from renewable energy or alternatively H2-rich gases from conventional steam reformers. As yet another alternative, HYFOR can run on H2-rich waste gases. Depending on the source of the hydrogen, this leads to a low or even zero CO2 footprint for the resulting direct-reduced iron. The plant features a modular design with a rated capacity of 250,000 tons per module and year, making it suitable for all sizes of steel plants. A pilot plant for testing purposes was started up in April 2021 at voestalpine Stahl Donawitz, Austria.
Works with low-quality ores
HYFOR both drastically reduces CO2 emissions and helps producers to effectively deal with the challenge of reduced iron-ore quality, which has become more acute as of late—resulting in an increased need to beneficiate the ores. Rising demand for iron-ore pellets for blast furnaces and direct-reduction plants has led to higher prices for iron ore, especially pellet premium. With HYFOR, producers will be able to use pellet-feed fine ore directly and benefit from the rising global supply of ultrafines.
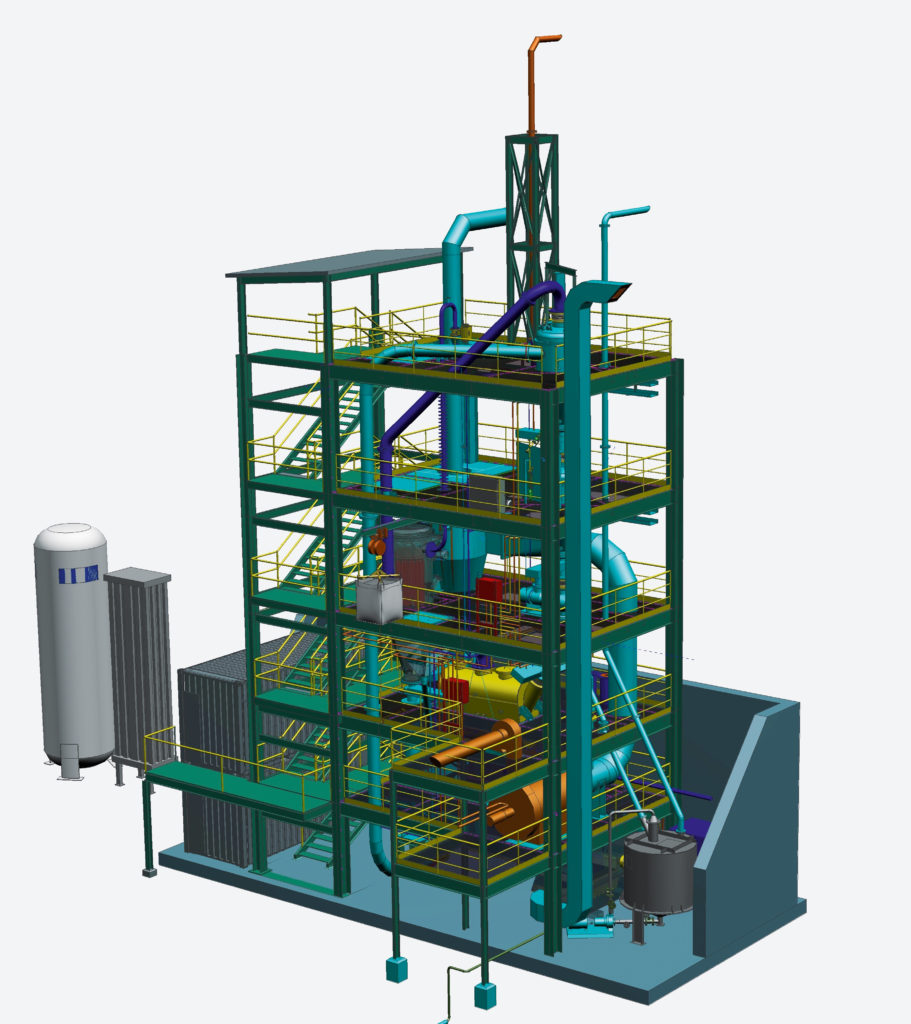
The pilot plant
The HYFOR pilot plant at voestalpine Donawitz consists of three parts: A preheating-oxidation unit, a gas-treatment plant, and the core—the novel and unique reduction unit. In the preheating-oxidation unit, fine-ore concentrate is heated to approximately 900°C and fed to the reduction unit. The reduction gas H2 is currently supplied over the fence from a gas supplier. A waste-heat recovery system that harnesses heat from the off-gas ensures optimal energy use and a dry-dedusting system takes care of dust emissions from the processes. The resulting hot direct-reduced iron (HDRI) leaves the reduction unit at a temperature of approximately 600°C and can subsequently be fed into an electric arc furnace (EAF) or used to produce hot-briquetted iron.
The purpose of the pilot plant is to provide practical evidence for this breakthrough process and to serve as a testing facility, collecting enough data to set up an industrial-scale plant at a later stage.
Hydrogen Electrolysis with Green Energy
As of today, most of the hydrogen for industrial use is produced by steam methane reformers (SMRs). Since the natural gas that feeds these reformers (methane—CH4) contains carbon, the resulting “gray” hydrogen causes a sizable amount of CO2 emissions. To fully decarbonize the process, hydrogen for ironmaking will have to be produced by electrolysis from water, using fossil-free energy.
Since the year 2000, more than 230 hydrogen-electrolysis projects (counting both those based on renewable as well as conventional sources of energy) have entered into operation around the world. Many of them are based in Europe, but several have been started or announced in Australia, China, and the Americas. Almost all have been at a scale of less than 10 MW, but a 20 MW plant has been inaugurated in Canada and lately there have been several proposals for plants at gigawatt scale — chief among them is the Asian Renewable Energy Hub project in Western Australia.
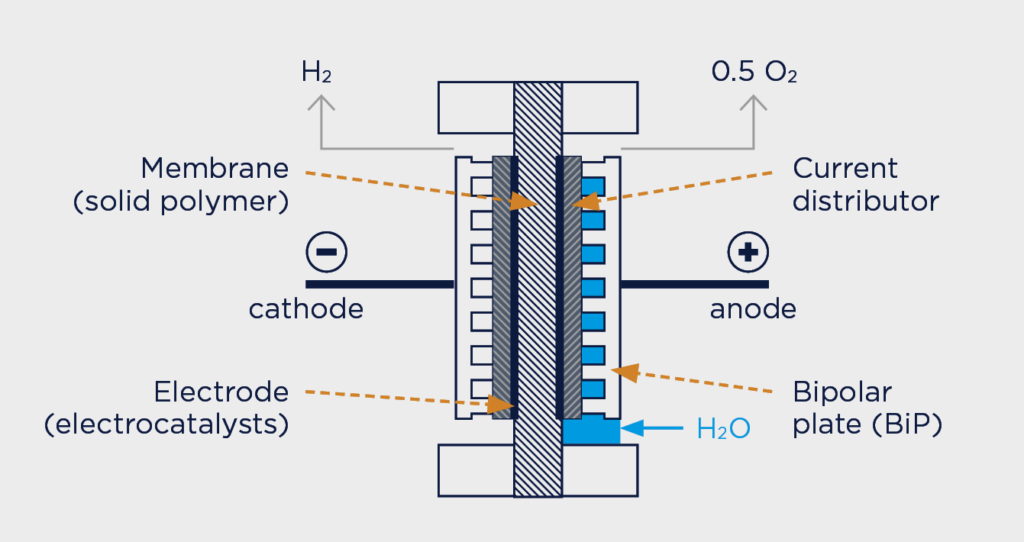
There are three main electrolysis processes that make all of this possible: alkaline, proton-exchange membrane (PEM), and high-temperature steam electrolysis. The most advanced technology at this point—and the one that most recent hydrogen-generation projects have favored, is PEM. It attaches electrodes on two sides of a solid polymer membrane, which acts as the electrolyte and as a separator to prevent the produced gases from mixing. Hydrogen ions form at the anode, pass through the membrane and combine with electrons from the cathode to form hydrogen gas.
The PEM-type electrolyzer has several advantages: It is highly efficient, features high power density, and has an extended dynamic operating range, allowing it to be directly coupled to renewable sources—because it can quickly react to changes in the electricity supply. Modules are available in the range of 3 to 100 MW, producing up to 20,000 Nm³ (or roughly 2 tons) of hydrogen per hour.



